
How to Join the Zantac Lawsuit Mass Tort | Zantac Settlement Guide
If you or a loved one has used Zantac or generic Ranitidine products and suffered health issues, including various types of cancer, you may be eligible to join the Zantac lawsuit mass tort. This guide will provide you with the essential steps to get involved, including understanding potential damages, identifying the defendants, and navigating the legal process.
Joining the Zantac Lawsuit Mass Tort

Overview
The Zantac Multidistrict Litigation (MDL 2924) was filed in the U.S. District Court: Southern District of Florida and consolidates thousands of cases brought against Zantac (Ranitidine) producers/manufacturers.
The Zantac lawsuit mass tort is a legal action involving thousands of plaintiffs who claim that Zantac, a popular heartburn medication, contains harmful levels of NDMA (N-nitrosodimethylamine), a chemical linked to several types of cancer. By joining this mass tort, individuals who have been adversely affected by Zantac use may seek compensation for their injuries.
Free Consultation Details
To determine if you qualify for the Zantac mass tort, it’s essential to consult with a legal expert. Many attorneys offer free consultations to evaluate your case and discuss your options. This initial consultation helps you understand whether you meet the criteria for joining the lawsuit and what steps you need to take.

Who Are The Defendants In The Zantac And Generic Ranitidine Lawsuits?
GlaxoSmithKline
GlaxoSmithKline (GSK) is a British pharmaceutical company and one of the primary defendants in the Zantac lawsuits. GSK manufactured and marketed Zantac and is accused of failing to adequately warn consumers about the potential cancer risks associated with the drug.
Sanofi
Sanofi S.A., a French multinational pharmaceutical company, is also a key defendant. Sanofi, formerly known as Sanofi-Aventis, played a significant role in the production and distribution of Zantac and is being held accountable for its alleged contributions to the NDMA contamination.
Boehringer Ingelheim
Boehringer Ingelheim, a German pharmaceutical company, is another defendant in these lawsuits. The company is accused of contributing to the manufacturing and distribution of Zantac without sufficiently addressing the cancer risks linked to NDMA.
What is the Potential Damage Caused by Zantac?
Overview of Potential Health Risks
Zantac’s potential health risks are primarily linked to its NDMA contamination. NDMA is a known carcinogen that can lead to various serious health conditions. Beyond cancer, Zantac use may be associated with several other health issues.
Specific Health Issues Linked to Zantac
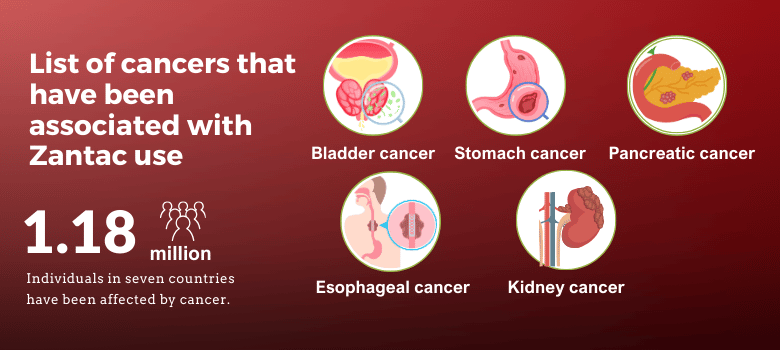
- Cancer: The most significant health risk associated with Zantac is cancer. Studies have linked NDMA to bladder cancer, stomach cancer, pancreatic cancer, breast cancer, prostate cancer, and brain cancer, among others.
- Kidney Problems: Zantac use has been connected to kidney issues, including kidney cancer and kidney failure, which can lead to severe health complications.
- Respiratory Issues: Some users have reported respiratory problems, such as coughing up mucus, which may indicate underlying issues caused by Zantac.
- Other Serious Conditions: Dark urine, easy bruising or bleeding, vision problems, weakness, joint pain, irregular heartbeat, chest pain, and loss of appetite are additional health concerns that have been associated with Zantac use.
Do I Qualify to Join the Zantac Lawsuit Mass Tort?
Eligibility Criteria
To qualify for the Zantac lawsuit mass tort, you generally need to meet the following criteria:
- Usage: You must have used Zantac or another product containing Ranitidine.
- Diagnosis: You must have been diagnosed with cancer or another serious health condition linked to Zantac.
- Timing: Ensure that your case falls within the statute of limitations for filing a lawsuit in your state.

Common Health Conditions Associated with Zantac Use
Common conditions reported by plaintiffs include various types of cancer (bladder, stomach, pancreatic, breast, prostate, brain) and other severe health issues like kidney failure and respiratory problems.
4 Steps to Take to Join the Zantac Lawsuit Mass Tort Settlement
Understanding the Civil Litigation Process
Before you file a Zantac lawsuit, familiarize yourself with the civil litigation process. Understanding the steps involved can help you prepare for what to expect and ensure that you follow the necessary procedures.
Mitigating Damages
To build a strong case, it is crucial to mitigate your damages. This involves seeking proper medical care, following your doctor’s advice, and minimizing any additional costs associated with your injuries.
Gathering Evidence
Collecting evidence is essential for supporting your claim. Relevant evidence includes:
- Receipts for Zantac purchases
- Medical records and bills
- Personal accounts of your health issues
- Photos or videos documenting your condition
- Proof of lost wages and other expenses
Hiring a Lawyer
Engage a personal injury attorney with experience in handling pharmaceutical lawsuits. A lawyer will help you gather evidence, establish liability, assess damages, and navigate the legal process.

Understanding the Statute of Limitations
Each state has a specific statute of limitations for filing a lawsuit. Typically, you have two years from the date of diagnosis to file a Zantac lawsuit. Consult with your attorney to ensure you file within the allowed time frame.
Determining Damages
Your attorney will help assess both economic and non-economic damages, including medical expenses, future care costs, pain and suffering, and loss of earning capacity. They will file a complaint seeking compensation to cover these damages.
The Zantac lawsuit highlights the vital need for pharmaceutical companies to ensure the safety of their products. The discovery of NDMA contamination in Zantac has raised significant health concerns, leading to legal actions that have affected countless individuals. If you or a loved one has taken Zantac and subsequently developed cancer or other severe health issues, it’s essential to understand your legal rights. Don’t wait—take action now to seek the justice and compensation you deserve. Reach out to a legal professional today to discuss your situation and begin the journey toward securing your future.
Baby Formula NEC
NEC Baby Formula Lawsuit: Everything You Need to Know in 2024

Parents of premature infants who developed necrotizing enterocolitis (NEC) after consuming certain baby formulas are seeking justice through lawsuits against major formula manufacturers like Similac and Enfamil. Studies have linked cow’s milk-based formulas to an increased risk of NEC in preterm babies, leading many families to take legal action.
In this article, we’ll break down what the NEC baby formula lawsuit is, the most recent updates, and what parents can do if their infant was affected.
Table of Contents
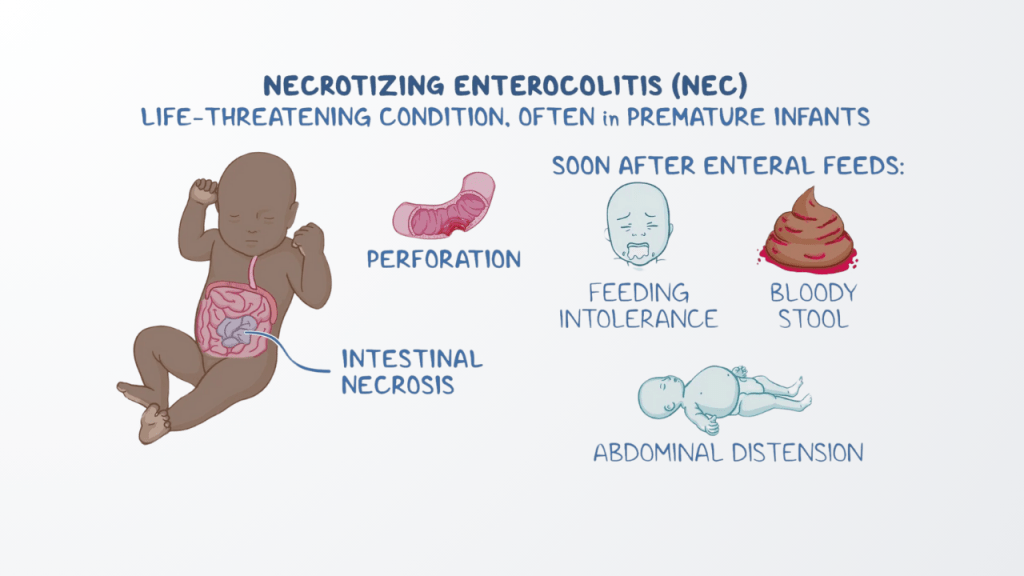
What is NEC and Why is it Dangerous?
- Necrotizing enterocolitis (NEC) is a serious intestinal disease that primarily affects premature and low birth weight infants. It causes inflammation and infection, which can lead to the destruction of the intestines. In severe cases, NEC can result in:
- Infections
- Intestinal perforation
- Sepsis
- Long-term health issues
- Death (in up to 30% of cases)
NEC symptoms can begin with feeding intolerance, and in many cases, babies with this condition require emergency surgery to remove the damaged part of the intestine.
Link Between NEC and Baby Formula
Several studies have shown a direct link between feeding premature infants cow’s milk-based formulas like Similac and Enfamil, and a higher risk of NEC. Babies fed these formulas were more likely to develop NEC compared to those who were fed breast milk.
In fact, research dating as far back as 1990 indicated that formula-fed preemies are 6 to 10 times more likely to develop NEC than those who are exclusively breastfed. More recent studies in 2009 and 2014 further confirmed that premature babies on human milk-based formulas or exclusively breastfed were 90% less likely to require surgery for NEC or die from the disease.
Baby Formulas Involved in NEC Lawsuits
While Similac and Enfamil are the most well-known baby formulas involved in these lawsuits, other cow’s milk formulas are also being scrutinized. Some brands mentioned in the lawsuits include:
- Avacare
- Baby’s Only
- Bobbie
- Earth’s Best
- Enfamil
- Gerber
- Happy Baby
- Holle
- Lebenswert
- Loulouka
- Kendamil
- Parent’s Choice
- PediaSure
- Similac
If your baby consumed any of these formulas in the hospital or after discharge and was later diagnosed with NEC, you may qualify to file an NEC baby formula lawsuit.
Recent Developments in the NEC Baby Formula Lawsuit
The legal battle against baby formula manufacturers continues to grow, and the most recent verdicts are promising for plaintiffs.
As of September 2024, there are 571 active NEC baby formula lawsuits pending under multidistrict litigation (MDL 3026), which is being overseen by U.S. District Judge Rebecca Pallmeyer in the Northern District of Illinois.
While no settlements or jury verdicts have been reached within the MDL, which primarily involves Similac and Enfamil lawsuits, state court trials have resulted in significant plaintiff victories. In March 2024, an Illinois state court awarded $60 million to the plaintiff, and in July 2024, a Missouri state court awarded $496 million in a separate case.
Judge Pallmeyer has tentatively scheduled the first MDL bellwether trial for May 2025.
We will continue to track court filings and collaborate with legal experts to keep you informed on major developments and critical updates in these lawsuits.
Baby Formula Litigation Timeline
July 2024
This month brought another significant victory for plaintiffs. A jury in St. Louis, Missouri, awarded $495 million to the family of a baby girl who developed necrotizing enterocolitis (NEC) after consuming Abbott’s premature infant formula in the NICU up until 2021. The infant suffered permanent neurological damage and will require lifelong care. This case marked the second NEC lawsuit to go to trial. The first trial, held in March 2024, resulted in a $60 million verdict against Reckitt.
June 2024
Judge Rebecca Pallmeyer issued a scheduling order, finally providing clarity on the potential timeline for the first bellwether trial. According to court documents, fact discovery is expected to conclude by early August 2024, followed by depositions and additional proceedings as the litigation moves toward trial. The first bellwether test trial is currently estimated to begin in May 2025. A prior trial in Illinois state court, which awarded $60 million to a mother whose child passed away from NEC, could set a positive precedent for plaintiffs as we approach the MDL trials. Whether defendants choose to continue litigation or consider a global settlement remains to be seen.
May 2024
Judge Pallmeyer ruled that plaintiffs must provide specific evidence demonstrating that an infant consumed a Mead Johnson product before the company can be named as a defendant in the lawsuit. Acceptable evidence may include medical records or feeding logs from birth.
March 2024
An Illinois jury awarded $60 million to the mother of a premature infant who died after developing NEC from consuming Mead Johnson’s Enfamil formula. Mead Johnson is a subsidiary of Reckitt Benckiser.
February 2024
Judge Pallmeyer scheduled several private hearings involving plaintiffs for March 2024. These motions were sealed from the public.
December 2023
At the close of 2023, four bellwether cases had been selected. However, trial dates had yet to be confirmed, with many expecting the first trial to occur sometime in 2024.
April 2023
Negotiations between lawyers continued regarding the scope and process of discovery for the upcoming trials.
March 2023
The court scheduled a Science Day for May 3, 2023, where both parties presented the medical and scientific issues relevant to NEC and the baby formula to the judge.
December 2022
The defendants selected four cases for bellwether test trials: Clarke, Cresap, Inman, and Mar.
October 2022
The plaintiffs also selected four bellwether cases for trial: Brown, Diggs, Lopez, and Koeth. Additionally, randomly selected cases for bellwether trials included McCarthy, Jacobs, Kelton, and Donaldson.
Why Are NEC Baby Formula Lawsuits Being Filed?
Lawsuits against manufacturers like Mead Johnson (Enfamil) and Abbott Laboratories (Similac) argue that these companies failed to warn consumers about the risks associated with feeding cow’s milk-based formulas to premature infants. Plaintiffs claim that:
- There were no warnings about the risk of NEC or death for preterm infants.
- The defendants knew or should have known that their products were dangerous for preemies but marketed them as safe.
- Misleading marketing campaigns gave doctors and parents the false impression that these formulas were safe for fragile newborns.
Who Qualifies to File an NEC Baby Formula Lawsuit?
If your baby was born prematurely, consumed a cow’s milk-based formula like Similac or Enfamil, and was later diagnosed with NEC, you may be eligible to file a lawsuit. Specific qualifications include:
- Your baby consumed cow’s milk protein formula.
- Your baby was born prematurely or with low birth weight.
- Your baby was diagnosed with NEC after using the formula.
Even if you’re unsure which formula your baby consumed, an attorney can help you gather medical records and feeding logs to determine if Similac or Enfamil was used. It’s important to keep any proof of purchase, formula packaging, or hospital records that can support your case.
Time Limits for Filing an NEC Baby Formula Lawsuit
Like all lawsuits, there are statutes of limitations that limit how long you have to file a claim. This time limit varies by state, so it’s crucial to speak with a lawyer as soon as possible to ensure your claim is filed in time. A knowledgeable NEC baby formula attorney can help preserve your right to compensation.
Finding the Right NEC Baby Formula Lawyer
Choosing the right lawyer to handle your case is a critical step in your lawsuit. Look for an attorney who has:
- Experience with mass torts and complex litigations against large corporations.
- A strong track record of winning verdicts and securing settlements in similar cases.
- Access to expert witnesses, medical professionals, and resources to effectively prove your case in court.
Many law firms offer free case evaluations, allowing you to explore your legal options without any upfront cost. You can easily get started by contacting a lawyer specializing in NEC lawsuits or mass torts.
Why Parents Are Filing NEC Baby Formula Lawsuits
Parents are pursuing lawsuits not only to seek compensation for medical bills, long-term care, and emotional pain but also to hold companies accountable for negligence. Many families hope that these lawsuits will lead to greater awareness about the risks of cow’s milk formulas and prevent future harm to premature infants.
Marie Smith, for example, filed a lawsuit after her daughter, Amirea, passed away from NEC at just two weeks old. She says, “No lawsuit will bring my daughter back, but I want to spread awareness and ensure no other parent has to go through the same nightmare.”
Have Enfamil and Similac Formulas Been Recalled?
While Enfamil and Similac have not been recalled due to their potential link to necrotizing enterocolitis (NEC), there have been isolated recalls related to other issues. These include incidents of product tampering and a significant recall in February 2022 due to concerns over bacterial contamination.
One tampering case involved reports from mothers who discovered that their Enfamil products had been replaced with flour. Another incident occurred when infants became ill, and some tragically passed away after consuming Enfamil, reportedly due to bacterial infections. However, after testing, the FDA determined that the formula was safe.
In February 2022, Abbott Nutrition recalled certain lots of Similac, EleCare, and Alimentum formulas produced at their Sturgis, Michigan facility. This recall came after four infants were diagnosed with Cronobacter sakazakii infections, and one baby contracted Salmonella Newport. Sadly, two of the infants passed away.
Following the recall, the FDA conducted a preliminary inspection (inspection report) in March 2022, revealing that Abbott lacked adequate process controls to prevent infant formula from being contaminated with microorganisms. In June 2022, the FDA received another report of a possible infant death linked to bacterial contamination. That same month, Abbott’s Sturgis plant resumed operations to help alleviate the baby formula shortage exacerbated by the recall.
Protecting Your Rights in the NEC Baby Formula Lawsuit
The NEC baby formula lawsuit is a complex legal matter with devastating consequences for families. If your child developed NEC after consuming a cow’s milk-based formula, it’s essential to explore your legal options. With recent victories in court and promising developments in the ongoing MDL, now is the time to take action.
By holding formula manufacturers accountable, parents are not only seeking justice for their own children but also helping to raise awareness of the risks that cow’s milk-based formulas pose to premature infants.
If you believe your family may be entitled to compensation, contact an experienced NEC baby formula lawyer today to discuss your case and find out what steps to take next.
Mass Torts Lawsuit
Zantac Lawsuit Health Risks, Legal Actions, and Settlement Updates 2024

Introduction
The Zantac lawsuit is one of the most significant legal actions in recent pharmaceutical history, involving thousands of plaintiffs who allege that the popular heartburn medication, Zantac, led to severe health complications, including cancer. This blog delves into the background of the Zantac lawsuit, the discovery of its contamination with a probable human carcinogen, and the legal and health implications for those affected.
Table of Contents

Overview of the Zantac Lawsuit
The Zantac lawsuit emerged after the discovery that the medication, which contains ranitidine, could form N-Nitrosodimethylamine (NDMA), a potent carcinogen, under certain conditions. NDMA is known to increase the risk of several types of cancer, leading to widespread recalls and thousands of lawsuits against manufacturers like Sanofi and Pfizer. The Zantac lawsuit seeks to hold these companies accountable for failing to warn consumers about the potential dangers of their product.
Background on Ranitidine and NDMA Contamination
Ranitidine, the active ingredient in Zantac, was originally hailed as a breakthrough in treating heartburn and acid reflux. However, studies revealed that ranitidine could degrade and form NDMA, especially when exposed to heat or stored for long periods. NDMA is classified as a probable human carcinogen, meaning it could cause cancer in humans. This contamination is at the heart of the Zantac lawsuit, as plaintiffs claim they were unknowingly exposed to a harmful substance.
What Led to the Zantac Lawsuits?
Discovery of NDMA in Zantac
The discovery of NDMA in Zantac was first made public in 2019 when an independent laboratory, Valisure, detected high levels of the carcinogen in the medication. This finding triggered widespread concern, leading to further investigations by regulatory bodies worldwide.
9 RANITIDINE PRODUCTS CONTAINING NDMA
These are the nine (9) most common Zantac products and generic Zantac products containing ranitidine:
- Zantac 150 Tablets
- Zantac 150 Maximum Strength
- Zantac 150 Maximum Strength Cool Mint
- Zantac 75 Tablets
- Wal-Zan 150
- Wal-Zan 75
- Heartburn Relief
- Acid Reducer
- Acid Control
FDA’s Response and Drug Recalls
The U.S. Food and Drug Administration (FDA) warning says NDMA was found at levels between 3,000 to 26,000 times higher than FDA approved standards.
Following Valisure’s findings, the FDA conducted its tests and confirmed the presence of NDMA in Zantac. In response, the FDA issued a series of warnings, and by April 2020, it requested the removal of all Zantac products from the market. This move prompted the filing of numerous Zantac lawsuits, as consumers who had used the drug for years feared they were at risk of developing cancer.
Public Reaction and Legal Actions
The news of NDMA contamination in Zantac sparked outrage among consumers and prompted immediate legal actions. Thousands of Zantac lawsuits were filed, with plaintiffs seeking compensation for medical expenses, pain and suffering, and punitive damages against the drug’s manufacturers. The public’s reaction highlighted the widespread trust in over-the-counter medications and the devastating consequences when that trust is broken.
Health Risks Linked to Zantac
Cancers Associated with Zantac Use
The central claim in the Zantac lawsuit is that the drug’s contamination with NDMA increases the risk of several types of cancer. These include, but are not limited to, bladder cancer, stomach cancer, liver cancer, pancreatic cancer, and esophageal cancer. Plaintiffs in the Zantac lawsuit argue that they developed these cancers as a direct result of taking the drug.
Other Health Issues: PPH and Crohn’s Disease
In addition to cancer, some plaintiffs have linked Zantac use to other serious health conditions such as Primary Pulmonary Hypertension (PPH) and Crohn’s Disease. While the evidence connecting Zantac to these conditions is less established, they are nonetheless significant issues raised in the Zantac lawsuit.
Scientific Evidence and Medical Studies
Numerous studies have been conducted to explore the link between ranitidine, NDMA, and cancer. While some studies show a strong correlation, others are less conclusive. However, the prevailing scientific consensus acknowledges the potential risks, forming the basis for the Zantac lawsuit. Ongoing research continues to investigate the full extent of these risks.

Notable Zantac Lawsuit Cases
Key Plaintiffs and Their Stories
Several high-profile cases have brought attention to the Zantac lawsuit. These cases often involve plaintiffs who took Zantac for years and later developed cancer. Their stories highlight the personal toll of the drug’s contamination and underscore the importance of holding pharmaceutical companies accountable.
High-Profile Settlements and Dismissals
Some Zantac lawsuits have already resulted in settlements, where companies like Sanofi and Pfizer agreed to compensate plaintiffs without admitting wrongdoing. However, not all cases have led to settlements; some have been dismissed, often due to lack of sufficient evidence linking Zantac use to cancer. These outcomes are crucial in shaping the broader legal landscape of the Zantac lawsuit.
Impact on Veterans and Military Personnel
Veterans and military personnel are a significant group affected by the Zantac lawsuit. Many veterans relied on Zantac for years to manage gastrointestinal issues, often prescribed by VA hospitals. As a result, they represent a large portion of the plaintiffs, with many suffering from cancers linked to NDMA exposure. Their inclusion in the Zantac lawsuit highlights the broader impact on those who served the country.
Legal Developments and Settlements
Timeline of Legal Actions and Court Decisions
The timeline of the Zantac lawsuit is marked by key legal milestones, including the initial discovery of NDMA, FDA warnings, and the filing of thousands of lawsuits. This section outlines the major court decisions and ongoing litigation efforts that continue to shape the Zantac lawsuit landscape.
Multidistrict Litigation (MDL) in Florida
Given the large number of Zantac lawsuits, many have been consolidated into a Multidistrict Litigation (MDL) in the Southern District of Florida. The MDL aims to streamline the legal process by handling pretrial proceedings collectively before returning cases to their original jurisdictions for trial. This consolidation is a critical development in the Zantac lawsuit, potentially leading to broader settlements or judgments.
Recent Settlements by Sanofi and Pfizer
Recently, some pharmaceutical companies involved in the Zantac lawsuit, including Sanofi and Pfizer, have reached settlements with plaintiffs. While the details of these settlements are often confidential, they represent a significant step in resolving some of the claims. These settlements could influence the outcome of other pending cases and the overall trajectory of the Zantac lawsuit.
Who Qualified for a Zantac Lawsuit?
Criteria for Eligibility
To qualify for a Zantac lawsuit, plaintiffs generally need to meet specific criteria. These include a confirmed diagnosis of cancer associated with NDMA exposure, a history of using Zantac, and evidence linking the medication to their illness. Eligibility criteria vary slightly depending on the jurisdiction and the specifics of each case.
Types of Cancer and Health Issues Covered
The Zantac lawsuit primarily covers cancers that have been scientifically linked to NDMA exposure, such as bladder, stomach, and liver cancers. However, some cases also involve other health issues, like PPH and Crohn’s Disease, which plaintiffs claim were exacerbated by Zantac use. Understanding the types of conditions covered is essential for those considering joining the Zantac lawsuit.
How to Determine Eligibility and Next Steps
Potential plaintiffs can determine their eligibility by consulting with a legal expert specializing in pharmaceutical litigation. These lawyers can review medical records, usage history, and other factors to assess the strength of a potential case. For those who qualify, the next steps often involve gathering evidence, filing a claim, and joining the Zantac lawsuit, either individually or as part of an MDL.

Understanding Zantac Litigation
Difference Between Individual Lawsuits and Class-Action
The Zantac lawsuit landscape includes both individual lawsuits and class-action suits. Individual lawsuits allow plaintiffs to pursue compensation based on their specific circumstances, while class-action suits consolidate many claims into one legal action. Understanding the differences can help plaintiffs decide which legal avenue best suits their situation.
Role of Lawyers and Legal Representation
Legal representation is crucial in the Zantac lawsuit, as experienced lawyers can navigate the complexities of pharmaceutical litigation. They assist plaintiffs in building a strong case, negotiating settlements, and ensuring that their rights are protected throughout the legal process. Choosing the right lawyer is a critical step in pursuing a Zantac lawsuit.
What Plaintiffs Need to Know About the Legal Process
The legal process for the Zantac lawsuit can be lengthy and complicated, involving multiple stages such as filing a claim, discovery, pretrial motions, and potentially a trial. Plaintiffs should be prepared for the time commitment and understand the possible outcomes, including settlements or court judgments. Staying informed and engaged in the process is key to a successful outcome.
Current Status of Zantac Lawsuits
Latest Updates on Pending Cases
As of now, the Zantac lawsuit continues to evolve, with new cases being filed and existing ones moving through the court system. Recent updates include ongoing trials, settlements, and decisions in the MDL. Staying informed about these developments is crucial for those involved or considering joining the Zantac lawsuit.
Future Outlook for Zantac Litigation
The future of the Zantac lawsuit remains uncertain, with potential for further settlements, additional lawsuits, and possibly new revelations about the drug’s safety. Legal experts predict that the litigation could continue for years, with significant implications for both plaintiffs and the pharmaceutical industry. Understanding the future outlook can help plaintiffs prepare for what lies ahead.
Resources for Affected Individuals
For those affected by the Zantac lawsuit, numerous resources are available, including legal assistance, support groups, and medical information. These resources can provide guidance on navigating the legal process, managing health concerns, and connecting with others in similar situations. Accessing these resources is an important step in dealing with the impact of Zantac use.

Conclusion
The Zantac lawsuit underscores the critical importance of holding pharmaceutical companies accountable for the safety of their products. The discovery of NDMA contamination in Zantac has led to widespread health concerns and legal actions, impacting thousands of individuals. If you or a loved one have taken Zantac and developed cancer or other serious health conditions, it’s crucial to explore your legal options. Don’t wait for further developments—seek the justice and compensation you deserve. Contact a legal professional today to discuss your case and take the first step toward securing your future.
Mass Torts Lawsuit
Zantac Lawsuit Statute of Limitations | What You Need to Know

Zantac Lawsuit Statute of Limitations
When considering legal action over Zantac (ranitidine), understanding the statute of limitations is crucial. This legal timeframe dictates how long you have to file a lawsuit after discovering an injury, such as cancer, linked to the medication. Here’s what you need to know about the statute of limitations for Zantac lawsuits.
Table of Contents
About the Zantac Lawsuit Statute of Limitations
The statute of limitations for Zantac lawsuits varies by state, generally ranging from one year to six years. This period starts from the date you discovered the injury or should have reasonably discovered it. Given that the FDA’s recall of Zantac occurred relatively recently, most plaintiffs should still be within this legal timeframe.
If you’re considering a lawsuit but are concerned that the statute of limitations may have expired, it’s worth noting that exceptions and extensions might apply. Consulting with a personal injury lawyer can provide guidance tailored to your specific situation.
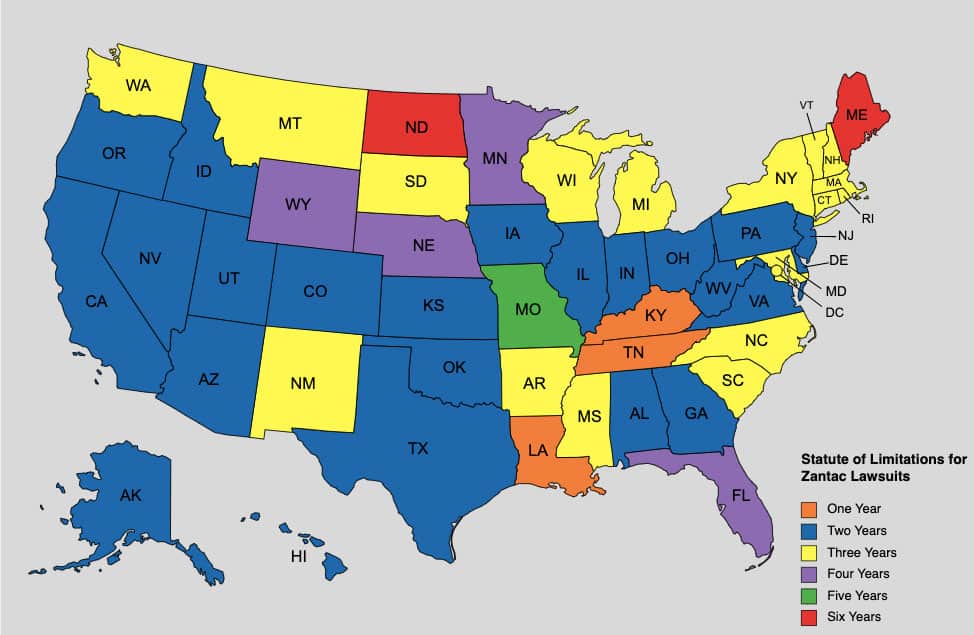
States with a 1 Year Statute of Limitations for Zantac Lawsuits
In these states, you generally have one year from the date of injury or diagnosis to file a lawsuit:
- Kentucky
- Louisiana
- Tennessee
States with a 2 Year Statute of Limitations for Zantac Lawsuits
If you reside in one of these states, you have two years from the injury or diagnosis to file your claim:
- Alabama
- Alaska
- Arizona
- California
- Colorado
- Delaware
- Georgia
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Nevada
- New Jersey
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Texas
- Utah
- Virginia
- West Virginia

States with a 3 Year Statute of Limitations for Zantac Lawsuits
In these states, you have three years from the date of injury or diagnosis to take legal action:
- Arkansas
- Connecticut
- Washington D.C.
- Maryland
- Massachusetts
- Michigan
- Mississippi
- Montana
- New Hampshire
- New Mexico
- New York
- North Carolina
- Rhode Island
- South Carolina
- South Dakota
- Vermont
- Washington
- Wisconsin

States with a 4 Year Statute of Limitations for Zantac Lawsuits
You generally have four years from the date of injury or diagnosis to file a lawsuit in these states:
- Florida
- Minnesota
- Nebraska
- Wyoming
States with a 5 Year Statute of Limitations for Zantac Lawsuits
In Missouri, you have five years from the date of injury or diagnosis to file your claim.
- Missouri
States with a 6 Year Statute of Limitations for Zantac Lawsuits
These states offer the longest timeframe, with six years allowed from the date of injury or diagnosis:
- Maine
- North Dakota

Additional Considerations
Since the FDA recall of Zantac happened on April 1, 2020, most claims should still be within the statute of limitations. However, exceptions and specific rules might apply based on individual circumstances and state laws. It’s essential to consult with a personal injury attorney to understand your legal options and ensure that your lawsuit is filed within the required timeframe.
Understanding the statute of limitations for your Zantac lawsuit is key to ensuring your rights are protected and that you have the opportunity to seek compensation for any harm caused by the medication.
-

 Roundup Weed Killer4 months ago
Roundup Weed Killer4 months agoEverything You Need to Know About Roundup Lawsuit
-

 Mass Torts Lawsuit4 months ago
Mass Torts Lawsuit4 months agoTop 5 Glyphosate Health Risks: Shocking Facts About Roundup Exposure You Need to Know
-
 Mass Torts Lawsuit4 months ago
Mass Torts Lawsuit4 months agoThe Roundup Non-Hodgkin’s Lymphoma Lawsuit: A Journey Through the Legal Battle
-

 Mass Torts Lawsuit4 months ago
Mass Torts Lawsuit4 months agoBest Law Firm for Roundup Lawsuits: Do You Qualify to File a Roundup Lawsuit?
-

 Mass Torts Lawsuit4 months ago
Mass Torts Lawsuit4 months agoRoundup Lawsuit 2024: What You Need to Know About Glyphosate Claims
-

 Mass Torts Lawsuit4 months ago
Mass Torts Lawsuit4 months agoHow Glyphosate in Roundup Affects Your Health.
-

 Mass Torts Lawsuit4 months ago
Mass Torts Lawsuit4 months agoMonsanto Glyphosate Lawsuit: Unveiling the Truth About Roundup and Cancer
-

 Roundup Weed Killer4 months ago
Roundup Weed Killer4 months agoThe Truth About Roundup Class Actions and MDL: 2024 Legal Insights




